Cell And Developmental Biology Laboratory
Members
| Name | Position | phone | |
|---|---|---|---|
| Petros Marangos | Associate Professor, Head of the Lab | pmaragos@uoi.gr | 2651007392 |
| Sandra Gonzalez Malagon | Post-Doctoral Researcher | sgonzalez@uoi.gr | 2651007344 |
| Konstantina Niaka | PhD student | niakonina@hotmail.com | 2651007344 |
| Kristy Agapitou | PhD student | kragapitou@yahoo.gr | 2651007344 |
Brief Description of the Laboratory
Our laboratory specializes in the examination of the cell cycle regulation and the DNA damage response during mammalian oocyte and early embryonic development. More specifically we determine cell cycle and DNA damage response regulators in the mouse oocyte, the pre-implantation mouse embryo and the mouse neural crest. The major directions of the research are:
- the examination of the DNA damage response in oocytes and pre-implantation embryos,
- the determination of cell cycle regulators in oocyte meiosis and the examination of their potential role in the regulation of oncogenesis,
- the cell cycle regulation and DNA damage response of mammalian neural crest stem cells.
The educational activity of the laboratory involves the teaching and logistical support of the practical exercises for the courses of Cell Biology, Developmental Biology, Reproductive Biology / Assisted Reproduction of the Department of Biological Applications and Technology.
DNA damage response and reproductive aging
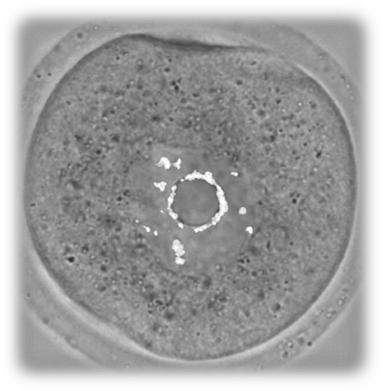
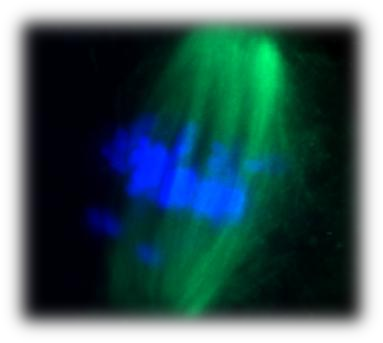
The increasing tendency of modern women to postpone childbearing has led to an increase in infertility, the development of pregnancies with chromosomal abnormalities and the pursuit for assisted reproductive treatments. Advanced maternal reproductive age has proven the main risk factor for genomic instability. A major cause of the occurrence of chromosomal abnormalities may be the inefficient response to DNA damage in oocytes. We have found that, although young oocytes lack a robust G2 DNA damage checkpoint (Marangos and Carroll, Current Biology, 2012), they establish a strong response to DNA damage in the first meiotic M-phase. This response is dependent on the activation of the spindle assembly checkpoint but also other important cell cycle regulators such as Mos/MAPK. However, aged oocytes do not show a strong response to DNA damage during meiosis I due to an inefficient spindle assembly checkpoint (Marangos et al., Nature Communications, 2015). We are currently examining the DNA damage response and especially DNA repair mechanisms in young and aged mouse and human oocytes. This work will allow the identification of the effects of DNA damage on oocyte meiosis, fertilisation and embryo development and will determine potential causes of human infertility.
|
|
| DNA damage in a Prophase-arrested mouse oocyte | Chromosome misalignment on an aged mouse oocyte spindle |
Oocyte cell cycle regulation: Linking Meiosis to Cancer
Cancer resistance to chemotherapeutic agents following prolonged exposure is a crucial problem in cancer therapy. The major reason for cancer drug resistance is the failure of damaged mitotic cells to arrest and die through apoptosis. We are examining oocyte-specific regulators as possible targets for developing new therapeutic methods to sustain drug sensitivity in cancer cells. It is known that oocyte-specific proteins, such as Mos, do not function only as meiotic regulators but are also implicated in cancer progression. In our laboratory we are examining the roles of oocyte-specific cytostatic factors in mouse oocyte M-phase, such as Mos
Cell cycle regulation and DNA damage response in neural crest development
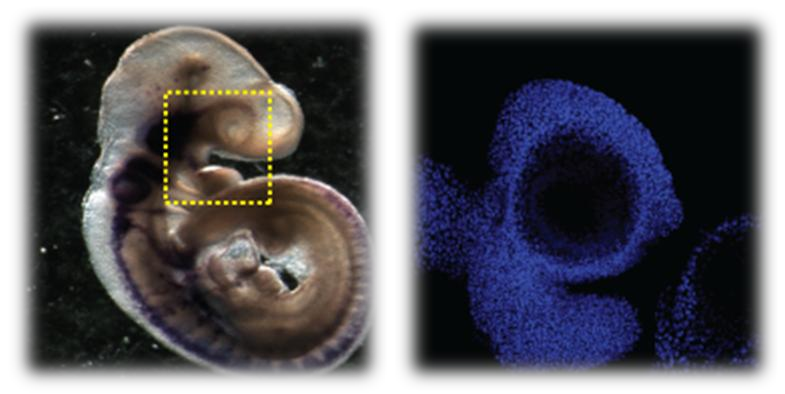
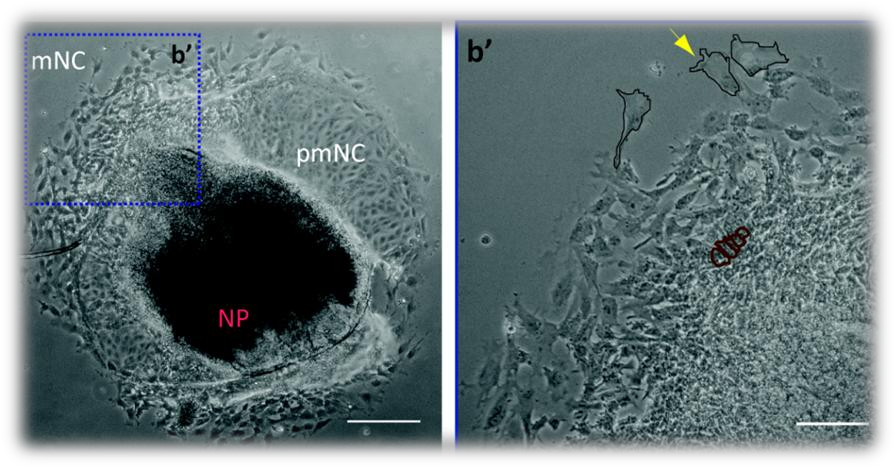
DNA damage is known to occur during embryogenesis where it promotes serious developmental abnormalities that may lead to severe disablement and even death. Moreover, some of the characteristics that cancer cells acquire are very similar to those found in stem cells involved in embryonic development. One of the most important cell populations during development is the neural crest stem cells (NCSCs). They not only act as progenitors for a vast variety of tissues but they possess the ability to migrate long distances, a characteristic also seen in metastatic cancer cells. It is known that NCSCs are responsible for serious developmental abnormalities that affect the head, the skin and the nervous, cardiac and digestive systems. The DNA damage response and cell cycle regulation of the mammalian neural crest has not yet been investigated. In our lab, we examine the regulation of the cell cycle and the DNA damage response in mammalian NCSCs. We investigate the mechanisms involved and the effects of DNA damage on the survival, migration and ifferentiation of NCSCs obtained from mouse embryos. We aim to determine whether DNA damage imposed on NCSCs is responsible for craniofacial abnormalities, embryonic tumors, pediatric and adult cancer and other developmental disorders. Furthermore, we investigate the cell cycle regulation during the different stages of neural crest development and specifically during migration and differentiation.
|
| Sox10 and DNA staining in a mouse E9.5 embryo |
|
| Mouse cranial Neural Crest explant in culture (NP:neural plate, pmNC:pre-migratory neural crest, mNC:migratory neural crest) |
Laboratory infrastructure
 Fully automated Nikon Eclipse Ti2-E including LED Lumencor Sola SE II 365
Fully automated Nikon Eclipse Ti2-E including LED Lumencor Sola SE II 365
 Zeiss Axiovert 100TV with Narishige micro-manipulators
Zeiss Axiovert 100TV with Narishige micro-manipulators
Selected publications
- Pailas A, Niaka K, Zorzompokou C, Marangos P (2022) The DNA Damage Response in Fully Grown Mammalian Oocytes. Cells. 11(5), 798; https://doi.org/10.3390/cells11050798
- Rémillard-Labrosse G., Dean N. L., Allais A., Mihajlović A. I., Jin S. G., Son W. Y., Chung J. T., Pansera M., Henderson S., Mahfoudh A., Steiner N., Agapitou K., Marangos P., Buckett W., Ligeti-Ruiter J., & FitzHarris G. (2020) Human oocytes harboring damaged DNA can complete meiosis I. Fertility and Sterility, 113(5), 1080–1089.e2.
- Gonzalez Malagon SG, Dobson L, Muñoz AML, Dawson M, Barrell W, Marangos P, Krause M, Liu KJ. (2019) Dissection, Culture and Analysis of Primary Cranial Neural Crest Cells from Mouse for the Study of Neural Crest Cell Delamination and Migration. J Vis Exp. (152):10.3791/60051.
- Nabti I, Grimes R, Sarna H, Marangos P, Carroll J. (2017) Maternal age-dependent APC/C-mediated decrease in securin causes premature sister chromatid separation in meiosis II. Nat Commun. 8:15346.
- Marangos P, Stevense M, Niaka K, Lagoudaki M, Nabti I, Jessberger R, Carroll J. (2015) DNA damage-induced metaphase I arrest is mediated by the Spindle Assembly Checkpoint and maternal age. Nat Commun. 6:8706.
- Marangos P, Carroll J. (2012) Oocytes progress beyond prophase in the presence of DNA damage. Curr Biol. 22:989-94.
- Marangos P, Carroll J. (2008) Securin regulates entry into M-phase by modulating the stability of cyclin B. Nat Cell Biol. 10:445-51.
